Clinical Applications: Sepsis
Central role of NETs in sepsis and septic shock
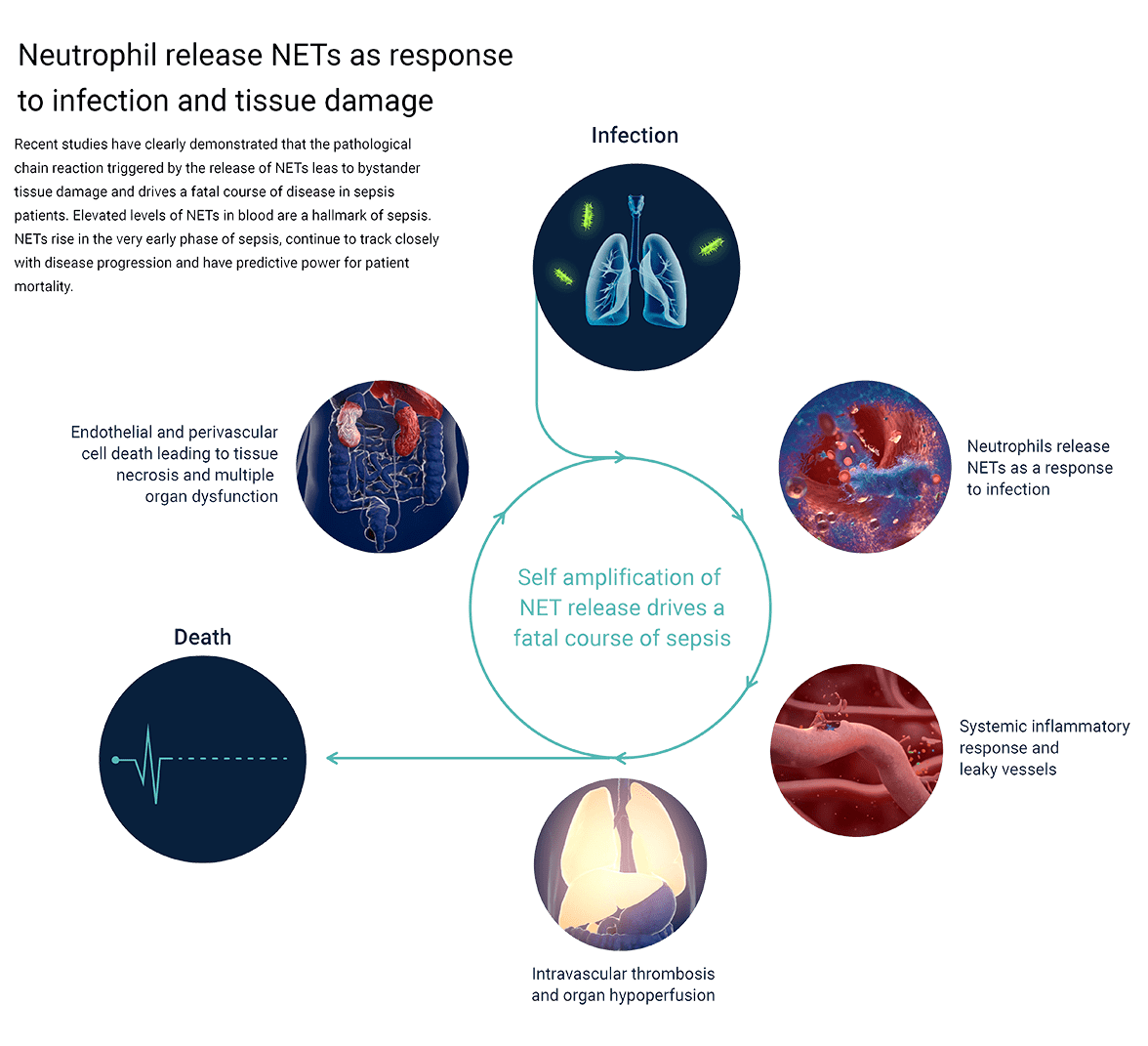
Image source: Infographic designed by Reciprocal Space www.reciprocal.space
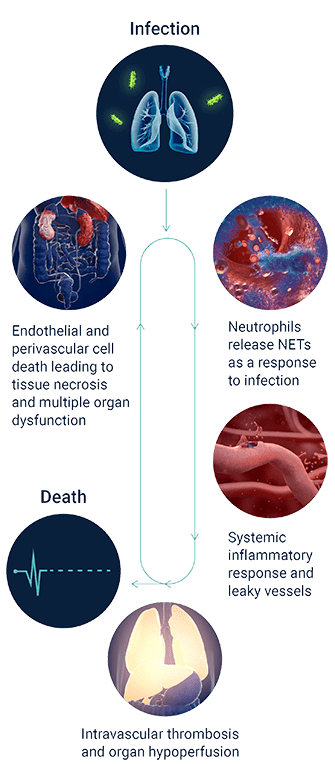
SEPSIS
Sepsis and Septic Shock: Preclinical Studies
Our preclincal studies of large animals with septic shock have confirmed that the removal of NETs with NucleoCapture lead to significant improvements in hemodynamic status and shock resolution.
Santersus AG has been granted Breakthrough Devices program for this indication from Q1 2022 by the FDA (The United States Food and Drug Administration).
The Breakthrough Devices Program is a voluntary program for certain medical devices and device-led combination products that provide for more effective treatment or diagnosis of life- threatening or irreversibly debilitating diseases or conditions.
Breakthrough Therapy designation description source: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/breakthrough-therapy
